
Peanut Allergy Treatment Market
Peanut Allergy Treatment Market - Global Industry Assessment & Forecast
Segments Covered
By Drug Class Antihistamines, Epinephrine, Immunotherapies, Other Drug Classes
By Route of Administration Oral, Injectable, Other Routes of Administration
By Distribution Channel Hospital Pharmacy, Retail Pharmacy, Other Distribution Channels
By Region North America, Europe, Asia Pacific, Latin America, Middle East & Africa
Snapshot
| 2023 | |
| 2024 - 2032 | |
| 2018 - 2022 | |
| USD 478.2 Million | |
| USD 1278.3 Million | |
| 11.6% | |
| Asia Pacific | |
| North America |
Customization Offered
Cross-segment Market Size and Analysis for Mentioned Segments
Additional Company Profiles (Upto 5 With No Cost)
Additional Countries (Apart From Mentioned Countries)
Country/Region-specific Report
Go To Market Strategy
Region Specific Market Dynamics
Region Level Market Share
Import Export Analysis
Production Analysis
Others Request Customization Speak To Analyst
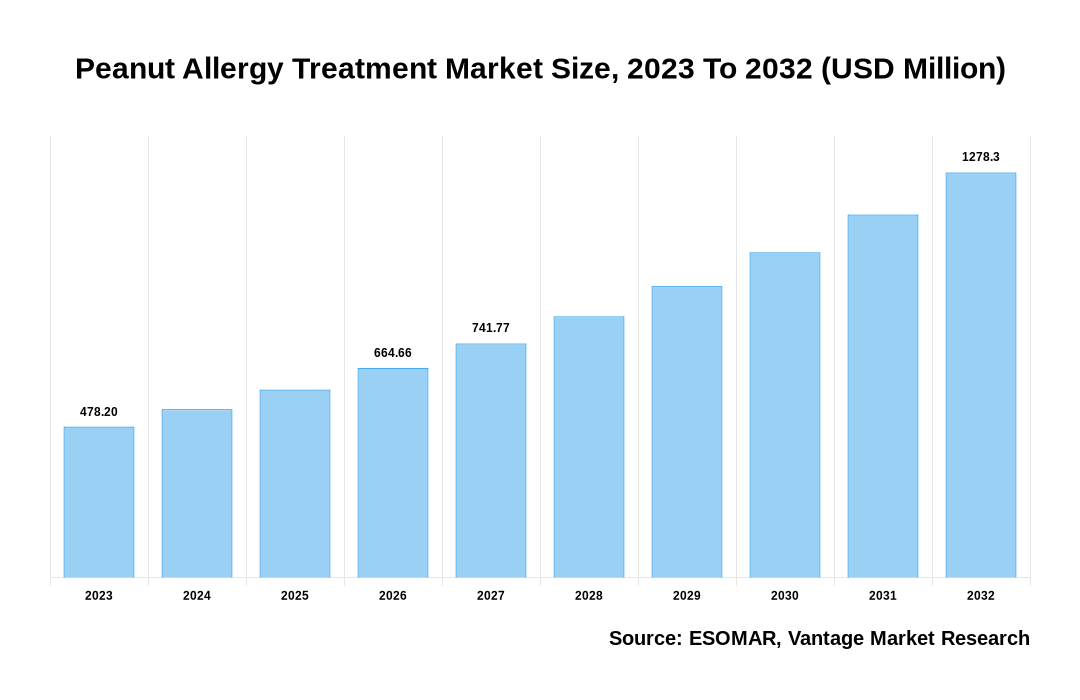
The global Peanut Allergy Treatment Market is valued at USD 478.2 Million in 2023 and is projected to reach a value of USD 1278.3 Million by 2032 at a CAGR (Compound Annual Growth Rate) of 11.6% between 2024 and 2032.
.
Key highlights of Peanut Allergy Treatment Market
- The North American region contributed the most considerable market growth, with a revenue share of 38.6% in 2023,
- The Asia Pacific area is predicted to increase at the fastest rate in the anticipated years, with a maximum CAGR of more than 12.3%,
- In 2023, the Epinephrine category contributed significantly to the Peanut Allergy Treatment market expansion with over 48.2% of the overall revenue share,
- In 2023, the Injectable category held a notable revenue share of 56.1% in the Peanut Allergy Treatment market,
- Based on the Distribution Channel segmentation, the Hospital Pharmacy segment accounted for have most potential market growth with a revenue share of 55.2% in 2023,
- The Peanut Allergy Treatment Market is propelled by the widespread impact of food allergies on 32 million Americans, with approximately 1 in 13 children affected, equivalent to 2 in every average-sized American classroom, according to findings from The Cleveland Clinic Foundation. A recent survey involving 4,075 respondents, encompassing both children and adults, indicated that roughly 38% reported experiencing at least one food-related allergic reaction annually, highlighting the pressing need for advancements in treatment solutions.
Peanut Allergy Treatment Market Size, 2023 To 2032 (USD Million)
AI (GPT) is here !!! Ask questions about Peanut Allergy Treatment Market
Peanut Allergy Treatment Market: Regional Overview
With a revenue share of 38.6% in 2023, North America led the Peanut Allergy Treatment market. This can be attributed to the rising number of people with peanut allergies in this region. About 1.6 million Americans suffer from a peanut allergy, and this number is continually growing, according to Food Allergy Research & Education (FARE). This rising prevalence has created a need for effective treatment options to manage and alleviate the symptoms associated with peanut allergies. In addition, the growing awareness about peanut allergies and their impact on individuals' quality of life contributes to the Peanut Allergy Treatment market's growth. Public health campaigns, educational programs, and increased media coverage have all contributed to a better understanding of peanut allergies among the general public and healthcare professionals. This increased awareness has expanded the number of individuals seeking treatment for their peanut allergy and has created a demand for innovative and effective treatment options.
U.S. Peanut Allergy Treatment Market Overview
The U.S. Peanut Allergy Treatment Market is valued at USD 175.2 Million in 2023 and is projected to reach a value of USD 390.2 Million by 2032 at a CAGR (Compound Annual Growth Rate) of 9.3% between 2024 and 2032. Advancements in treatment options have played a significant role in driving the growth of the Peanut Allergy Treatment market in the U.S. In recent years, various treatment approaches for peanut allergies have emerged, including oral immunotherapy (OIT), sublingual immunotherapy (SLIT), and epicutaneous immunotherapy (EPIT). These treatments aim to desensitize individuals to peanuts by gradually exposing them to small amounts of peanut protein, thereby reducing the severity of allergic reactions. For instance, Palforzia, developed by Aimmune Therapeutics, is the first FDA-approved oral immunotherapy for Peanut Allergy Treatment. It is an essential milestone in the market as it provides a treatment option for individuals with peanut allergies, helping them build tolerance to peanuts. This treatment involves a carefully controlled increase in the doses of peanut protein exposed to individuals over time under the supervision of a healthcare professional.
The global Peanut Allergy Treatment market can be categorized as Drug Class, Route of Administration, Distribution Channel, and Region.
| Parameter | Details |
|---|---|
| Segments Covered |
By Drug Class
By Route of Administration
By Distribution Channel
By Region
|
| Regions & Countries Covered |
|
| Companies Covered |
|
| Report Coverage | Market growth drivers, restraints, opportunities, Porter’s five forces analysis, PEST analysis, value chain analysis, regulatory landscape, technology landscape, patent analysis, market attractiveness analysis by segments and North America, company market share analysis, and COVID-19 impact analysis |
| Pricing and purchase options | Avail of customized purchase options to meet your exact research needs. Explore purchase options |
Peanut Allergy Treatment Market: Drug Class Overview
In 2023, the epinephrine segment led the market and accounted for a revenue share of 48.2%. Based on Drug Class, the Peanut Allergy Treatment market is divided into Antihistamines, Epinephrine, Immunotherapies, and Others. A popular emergency treatment for severe allergic responses, or anaphylaxis, is epinephrine. One of the most prevalent and possibly life-threatening food allergies is peanut allergy, which can result in anaphylactic reactions when a person with a peanut allergy is exposed to peanuts or anything containing peanuts. According to the CDC (Centers for Disease Control and Prevention), the prevalence of food allergies in children has increased recently, and one of the most common reactions is to peanuts. African American adults and children are some of the population most likely to be diagnosed with a food allergy, accounting for over 6% of cases in the United States among adults and children overall. This increasing prevalence has increased demand for Peanut Allergy Treatments, including epinephrine.
Peanut Allergy Treatment Market: Route of Administration Overview
The Injectable segment accounted for the most significant revenue share of 56.1% in 2023. By Route of Administration, the Peanut Allergy Treatment market is classified into Oral, Injectable, and Others. Injectable immunotherapy involves a series of injections containing gradually increasing amounts of peanut allergens. These injections help the immune system become desensitized to peanuts, reducing the severity of allergic reactions. According to the trial carried out by Stanford University in California, a single injection of an antibody called etokimab may halt peanut allergy for at least two weeks. The most effective and recommended way to administer epinephrine is through an intramuscular injection into the thigh muscle. This route ensures rapid absorption and distribution of the medication, leading to quicker relief of anaphylactic symptoms. For instance, if someone who has a severe allergy to peanuts consumes one through accidentally and develops an allergic reaction that could be life-threatening, they would experience anaphylaxis. In this case, a healthcare professional would immediately administer an IM injection of epinephrine into the lateral thigh to deliver the medication quickly.

Peanut Allergy Treatment Market: Distribution Channel Overview
The hospital pharmacy segment accounted for the significant growth of the Peanut Allergy Treatment market with a revenue share of 55.2% in 2023. Based on the Distribution Channel, the Peanut Allergy Treatment market is classified into Hospital Pharmacy, Retail Pharmacy, and Others. Hospital pharmacies have the infrastructure and expertise to handle complex medication regimens and provide personalized care. Peanut Allergy Treatment often involves multiple medications, such as antihistamines, epinephrine auto-injectors, and immunotherapy drugs. Hospital pharmacists can create personalized prescription therapies, check for drug interactions, and counsel patients on appropriate use and possible side effects. Furthermore, hospital pharmacies have established relationships with healthcare providers, including allergists and immunologists, who refer patients to them for specific Peanut Allergy Treatments. Hospital pharmacies, by guaranteeing the availability of prescribed medications and allowing communication between various healthcare specialists, are essential parts of promoting and organizing patient care in these collaborative healthcare settings.
Key Trends
- Peanut allergies are becoming more prevalent globally, particularly in developed countries. This trend drives the demand for effective treatments to manage peanut allergies and prevent severe allergic reactions. According to the FDA, about one million children in the United States suffer from peanut allergies, and only one in five of them will outgrow it.
- There is a growing awareness of peanut allergies among individuals and healthcare providers. This increased awareness is leading to better diagnosis rates, which, in turn, fuels the demand for Peanut Allergy Treatments.
- Oral immunotherapy is emerging as a promising treatment option for peanut allergies. It involves gradually introducing small peanut allergens to the patient's diet to build tolerance. Oral immunotherapy medicines are being developed by a number of businesses, notably Aimmune Therapeutics, whose AR101 has FDA approval.
- Significant advancements have been made in managing allergic reactions caused by peanut allergies. Newer technologies and devices, such as epinephrine auto-injectors and wearable allergic reaction detection devices, are becoming more sophisticated and user-friendly.
- Researchers are exploring alternative treatment strategies for peanut allergies, such as sublingual immunotherapy and monoclonal antibody treatments. The goal of these therapies is to minimize the possibility of severe allergic reactions by desensitizing patients to peanut allergens.
Premium Insights
The NIH (National Institutes of Health) estimates that 3 million Americans, or 1.1% of the overall population, suffer from a peanut allergy, which presents a significant growth potential for the Peanut Allergy Treatment market. According to data published in the Journal of Allergy and Clinical Immunology, 1.8% of US adults with significant evidence of a peanut allergy and 2.9% of self-reporting people have a peanut allergy. It is worth noting that over 17% of adults with peanut allergy developed it in adulthood. Furthermore, there are differences between those who created the allergy as children and those who developed it as adults. In the former group, 75.4% of adults were diagnosed by a physician, compared to 58.9% in the latter group. Moreover, it is noteworthy that adults with a childhood-onset peanut allergy are more likely to use an epinephrine autoinjector (48% vs.35%) and to have a current prescription for epinephrine (56% vs. 44%). These detailed insights highlight the need for personalized treatments that address the diverse demographic and onset patterns within the peanut-allergic population, presenting a compelling opportunity for advancements in the Peanut Allergy Treatment Market.


Report Coverage & Deliverables
Get Access Now
Track market trends LIVE & outsmart rivals with our Premium Data Intel Tool: Vantage Point
Market Dynamics
Advancements in Peanut Allergy Treatments Addressing a Growing Health Crisis
The Cleveland Clinic Foundation has revealed that 32 million Americans, including 1 in 13 children or 2 in every average-sized American classroom, suffer from food allergies, highlighting a critical need that is driving growth in the Peanut Allergy Treatment Market. Recent surveys have also revealed that 38% of respondents, both children and adults, experience at least one food-related allergic reaction yearly. To address this urgent demand, researchers at UT Southwestern Medical Center have conducted a clinical trial and discovered that administering low doses of under-the-tongue immunotherapy can safely desensitize children aged 1 to 4 years to peanut allergies. This breakthrough offers the potential for a long-term immunologic response. It represents a significant advancement in managing the life-threatening nature of peanut allergies, which affect up to 2% of children in Western countries and often persist into adulthood. The progress in immunotherapy is a driving force in the Peanut Allergy Treatment Market and brings hope for effective interventions in managing this widespread and potentially fatal condition.
Oral Immunotherapy's Pivotal Role in Peanut Allergy Treatment Market
The Peanut Allergy Treatment Market is undergoing significant changes due to the emergence of oral immunotherapy. The US Food and Drug Administration (FDA) authorized PTAH (formerly known as AR101; PalforziaTM, Aimmune Therapeutics), a revolutionary peanut-derived oral immunotherapy medication, in January 2020 for the treatment of childhood peanut allergy. Being the first FDA-approved medication based on safety and efficacy data from clinical trials including over 700 individuals with peanut allergies, its approval represents a significant milestone. In allergy procedures, peanut oral immunotherapy (POIT) is becoming more and more common. It uses both store-bought and FDA-approved peanut products. Notably, POIT is being accepted for use in pre-schoolers due to evidence showing its safety, feasibility, and distinct advantages. The market is seeing an increase in the number of new therapeutic interventions being studied using various approaches and potential mechanisms. While other forms of immunotherapy are also being investigated, biologics are emerging as adjunctive treatments to POIT and standalone therapies, adding to the possibilities and momentum in the Peanut Allergy Treatment Market.
Competitive Landscape
Key players in the Peanut Allergy Treatment Market have recently made significant advancements. ADP101 can be a safe and useful treatment for kids with common food allergies, according to encouraging study data from Alladapt Immunotherapeutics Inc. This introduces a new player with innovative approaches. DBV Technologies also made a breakthrough in food allergy treatment with their trial on Viaskin Peanut in children aged 1 to 3 years. This provides a novel and potentially effective option for managing food allergies in young children. These developments show how competitive the industry is with innovative solutions and a wider variety of available treatments.
The key players in the global Peanut Allergy Treatment market include - Alladapt Immunotherapeutics Inc. (U.S.), DBV Technologies (France), Aimmune Therapeutics Inc. (U.S.), Aravax Pty Ltd. (Australia), Sanofi (France), Vedanta Biosciences Inc. (U.S.), Regeneron Pharmaceuticals Inc. (U.S.), Teva Pharmaceuticals Industries Ltd. (Israel), Prota Therapeutics (Australia) among others.
Recent Developments
- In January 2024, Regeneron Pharmaceuticals and Sanofi have received approval from the U.S. Food and Drug Administration for Dupixent (dupilumab) to be used as a treatment for eosinophilic esophagitis (EoE) in pediatric patients aged 1 to 11 years, weighing at least 15 kg.
- In November 2023, Alladapt Immunotherapeutics, Inc. received approval from the FDA to fast-track the development of ADP101, a new treatment for food allergies. ADP101 is an oral medication that aims to treat allergies to one or more major food allergens. It is currently the most advanced candidate for this type of treatment.
- In November 2023, DBV Technologies shared encouraging initial findings from their ongoing study called EPOPEX. This study aims to evaluate the effectiveness of their product, Viaskin Peanut 250 µg, in children aged 1 to 3 years who have a peanut allergy. After the participants completed their involvement in the previous study called EPITOPE, they were allowed to join EPOPEX. By doing so, they would receive a total of three years of treatment with Viaskin Peanut.
FAQ
Frequently Asked Question
What is the global demand for Peanut Allergy Treatment in terms of revenue?
-
The global Peanut Allergy Treatment valued at USD 478.2 Million in 2023 and is expected to reach USD 1278.3 Million in 2032 growing at a CAGR of 11.6%.
Which are the prominent players in the market?
-
The prominent players in the market are Alladapt Immunotherapeutics Inc. (U.S.), DBV Technologies (France), Aimmune Therapeutics Inc. (U.S.), Aravax Pty Ltd. (Australia), Sanofi (France), Vedanta Biosciences Inc. (U.S.), Regeneron Pharmaceuticals Inc. (U.S.), Teva Pharmaceuticals Industries Ltd. (Israel), Prota Therapeutics (Australia).
At what CAGR is the market projected to grow within the forecast period?
-
The market is project to grow at a CAGR of 11.6% between 2024 and 2032.
What are the driving factors fueling the growth of the market.
-
The driving factors of the Peanut Allergy Treatment include
Which region accounted for the largest share in the market?
-
North America was the leading regional segment of the Peanut Allergy Treatment in 2023.



 Vantage Market
Research | 09-Apr-2024
Vantage Market
Research | 09-Apr-2024
