
E Clinical Trials Solutions Suite Technologies Market
E Clinical Trials Solutions Suite Technologies Market - Global Industry Assessment & Forecast
Segments Covered
By Product Clinical Data Management Systems (CDMS), Clinical Trial Management Systems (CTMS), Randomization and Trial Supply Management (RTMS), Electronic Clinical Outcome Assessment (eCOA), Electronic Data Capture (EDC), Electronic Patient-reported Outcomes (ePRO), Clinical Data Integration Platforms
By Delivery Model Web- Based (On-Demand) Model, Licensed Enterprise (On-Premise) Model, Cloud- Based
By Development Phase Phase I, Phase II, Phase III, Phase IV
By End User Pharmaceutical and Biopharmaceutical Companies, Contract Research Organizations(CROs), Hospitals/ Healthcare Providers, Medical Device Manufacturers, Academic Research Institutions
By Region North America, Asia Pacific, Europe, Latin America, Middle East & Africa
Snapshot
| 2022 | |
| 2023 - 2030 | |
| 2017 - 2021 | |
| USD 6991.90 Million | |
| USD 16456.43 Million | |
| 12.90% | |
| Asia Pacific | |
| North America |
Customization Offered
Cross-segment Market Size and Analysis for Mentioned Segments
Additional Company Profiles (Upto 5 With No Cost)
Additional Countries (Apart From Mentioned Countries)
Country/Region-specific Report
Go To Market Strategy
Region Specific Market Dynamics
Region Level Market Share
Import Export Analysis
Production Analysis
Others Request Customization Speak To Analyst
Market Synopsis:
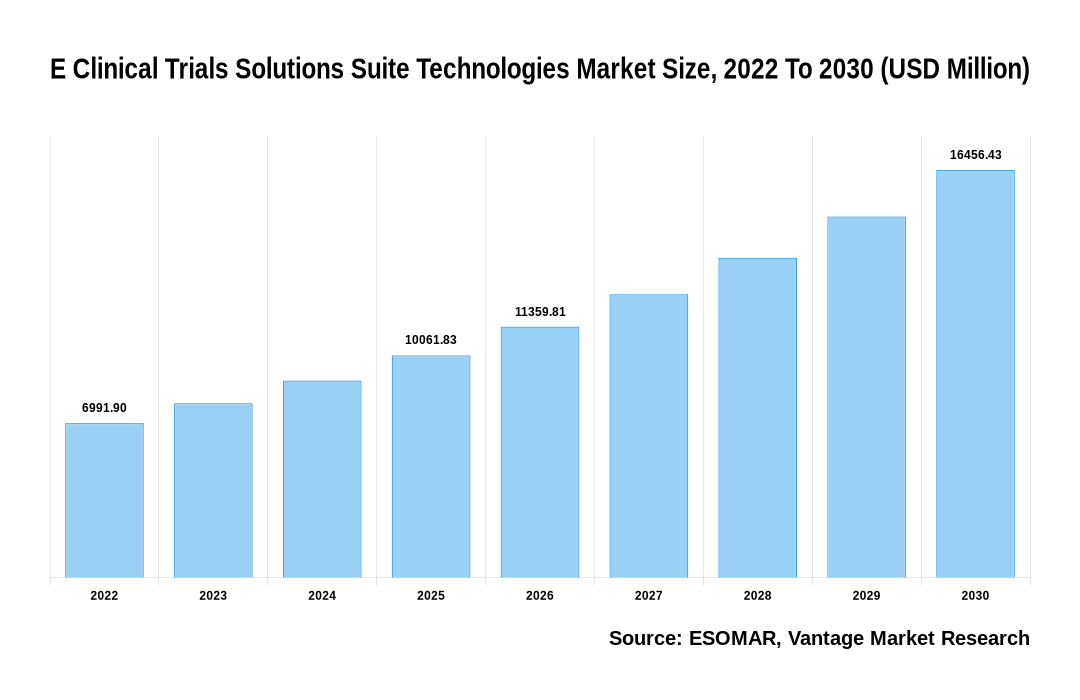
The Global E Clinical Trials Solutions Suite Technologies Market is valued at USD 6991.90 Million in 2022 and is projected to reach a value of USD 16456.43 Million by 2030 at a Compound Annual Growth Rate (CAGR) of 12.90% over the forecast period.
The E Clinical Trials Solutions Suite Technologies market is witnessing growth owing to the regulatory requirements associated with the clinical research studies and the increasing operational costs. Moreover, the growing research and development expenditure on the development of drugs by pharma-biotech companies are the prime factors expected to drive the growth of the E Clinical Trials Solutions Suite Technologies market in the years to come. The E Clinical Trials Solutions Suite Technologies manage the expertise and clinical technologies to enable acceleration of the clinical development process. The software helps to manage, record data, maintain and track the deadlines, and various E Clinical Trials Solutions Suite Technologies include randomization and trial supply management (RTSM), Clinical trial management system (CTSM), and Clinical data management system (CDMS) which are driving the growth of the e-clinical solution market. The growing adoption of novel software solutions in clinical research is a major factor fueling the growth of the E Clinical Trials Solutions Suite Technologies market during the forecast period. The rising clinical research activities in the developing countries are further expected to enhance the E Clinical Trials Solutions Suite Technologies market during the forecast period. The rising clinical research activities in the developing Asian countries and the greater outsourcing of the clinical trials processes to CROs are providing key opportunities for the growth of the E Clinical Trials Solutions Suite Technologies market in the upcoming years. However, the dearth of skilled professionals to operate E Clinical Trials Solutions Suite Technologies is limiting the growth of the market during the forecast period.
E Clinical Trials Solutions Suite Technologies Market Size, 2022 To 2030 (USD Million)
AI (GPT) is here !!! Ask questions about E Clinical Trials Solutions Suite Technologies Market
Market Segmentation:


Report Coverage & Deliverables
- Real-Time Data Updates:
- Competitor Benchmarking
- Market Trends Heatmap
- Custom Research Queries
- Market Sentiment Analysis
- Demographic and Geographic Insights
Get Access Now
The Web-Based Delivery Segment is Expected to Grow at the Highest CAGR During the Forecast Period:
The web-hosted segment is projected to grow at the highest Compound Annual Growth Rate (CAGR) during the forecast period. The growth of this web-based delivery segment is due to the cost reductions, and fast data recovery. Web-hosted products are effortlessly personalized, due to which providers can personalize the presentation of data for different user groups. In addition, these products include a high level of interoperability. However, the web-based delivery segment is dignified to maintain its position throughout the forecast period.
The Pharmaceutical End User Holds Largest Share in the E Clinical Trials Solutions Suite Technologies Market:
The growth of the pharmaceutical segment is primarily attributed to the increasing adoption of E Clinical Trials Solutions Suite Technologies by scientist that offers better clinical trials and efficient research. Moreover, such software solutions authorize biotechnology and pharmaceutical companies to classify bureaucratic bottlenecks associated with the clinical trials, this is driving the demand for E Clinical Trials Solutions Suite Technologies among researchers in the biotechnology and pharmaceutical companies for clinical research programs.
North America Region is Expected to Dominate the E Clinical Trials Solutions Suite Technologies Market During the Forecast Period:
The growth of this region is mainly attributed to the early adoption of technology united with many years of research and development in the pharmaceutical sector. Besides incentive programs introduced by the government bodies along with the enormous investments from the private sector are expected to drive the e-clinical solution market growth in North America during the forecast period.
Asia Pacific region is expected to grow at the highest Compound Annual Growth Rate (CAGR) during the forecast period. The growth of Asia Pacific is primarily attributed to fewer restrictions for clinical trials and the rising government funding united with prime clinical studies being outsourced to the technologically rich countries and regions which have pushed the e-clinical market in the Asia Pacific region by an enormous margin.
Competitive Landscape:
The E Clinical Trials Solutions Suite Technologies market is very competitive and continuously growing owing to the greater technology adoption and smaller and medium-sized sponsors. Some of the major key players in the market are Oracle Corporation (US), Medidata Solutions Inc. (US), Parexel International Corporation (US), BioClinica Inc. (US), Signant Health (US), Datatrak International Inc. (US), ERT (US), eClinical Solutions Inc. (US), MaxisIT Inc. (US), Bio-Optronics Inc. (US), Merge Healthcare Incorporated (US), and OmniComm Systems Inc. (US)..
E Clinical Trials Solutions Suite Technologies Market is Segmented as Follows:
Parameter
Details
Segments Covered
By Product
By Delivery Model
By Development Phase
By End User
By Region
Regions & Countries Covered
Companies Covered
Report Coverage
Market growth drivers, restraints, opportunities, Porter’s five forces analysis, PEST
analysis, value chain analysis, regulatory landscape, technology landscape, patent analysis, market
attractiveness analysis by segments and North America, company market share analysis, and COVID-19
impact analysis
Pricing and purchase options
Avail of customized purchase options to meet your exact research needs. Explore purchase options
E Clinical Trials Solutions Suite Technologies Market is Tabulated as follows:
FAQ
Frequently Asked Question
What is the global demand for E Clinical Trials Solutions Suite Technologies in terms of revenue?
-
The global E Clinical Trials Solutions Suite Technologies valued at USD 6991.90 Million in 2022 and is expected to reach USD 16456.43 Million in 2030 growing at a CAGR of 12.90%.
Which are the prominent players in the market?
-
The prominent players in the market are Oracle Corporation (US), Medidata Solutions Inc. (US), Parexel International Corporation (US), BioClinica Inc. (US), Signant Health (US), Datatrak International Inc. (US), ERT (US), eClinical Solutions Inc. (US), MaxisIT Inc. (US), Bio-Optronics Inc. (US), Merge Healthcare Incorporated (US), and OmniComm Systems Inc. (US)..
At what CAGR is the market projected to grow within the forecast period?
-
The market is project to grow at a CAGR of 12.90% between 2023 and 2030.
What are the driving factors fueling the growth of the market.
-
The driving factors of the E Clinical Trials Solutions Suite Technologies include
- Advantages of eClinical solutions
Which region accounted for the largest share in the market?
-
North America was the leading regional segment of the E Clinical Trials Solutions Suite Technologies in 2022.



