
Cancer Immunotherapy Market
Cancer Immunotherapy Market - Global Industry Assessment & Forecast
Segments Covered
By Product Monoclonal Antibodies, Immunomodulators, Oncolytic Viral Therapies & Cancer Vaccines
By Application Lung Cancer, Breast Cancer, Colorectal Cancer, Melanoma, Prostate Cancer, Head & Neck Cancer, Ovarian Cancer, Pancreatic Cancer, Other Cancers
By Distribution Channel Hospital Pharmacy, Retail Pharmacy, Online Pharmacy
By End Use Hospitals & Clinics, Cancer Research Centers, Other End Uses
By Region North America, Europe, Asia Pacific, Latin America, Middle East & Africa
Snapshot
| 2023 | |
| 2024 - 2032 | |
| 2018 - 2022 | |
| USD 121.2 Billion | |
| USD 245.37 Billion | |
| 8.15% | |
| Asia Pacific | |
| North America |
Customization Offered
Cross-segment Market Size and Analysis for Mentioned Segments
Additional Company Profiles (Upto 5 With No Cost)
Additional Countries (Apart From Mentioned Countries)
Country/Region-specific Report
Go To Market Strategy
Region Specific Market Dynamics
Region Level Market Share
Import Export Analysis
Production Analysis
Others Request Customization Speak To Analyst
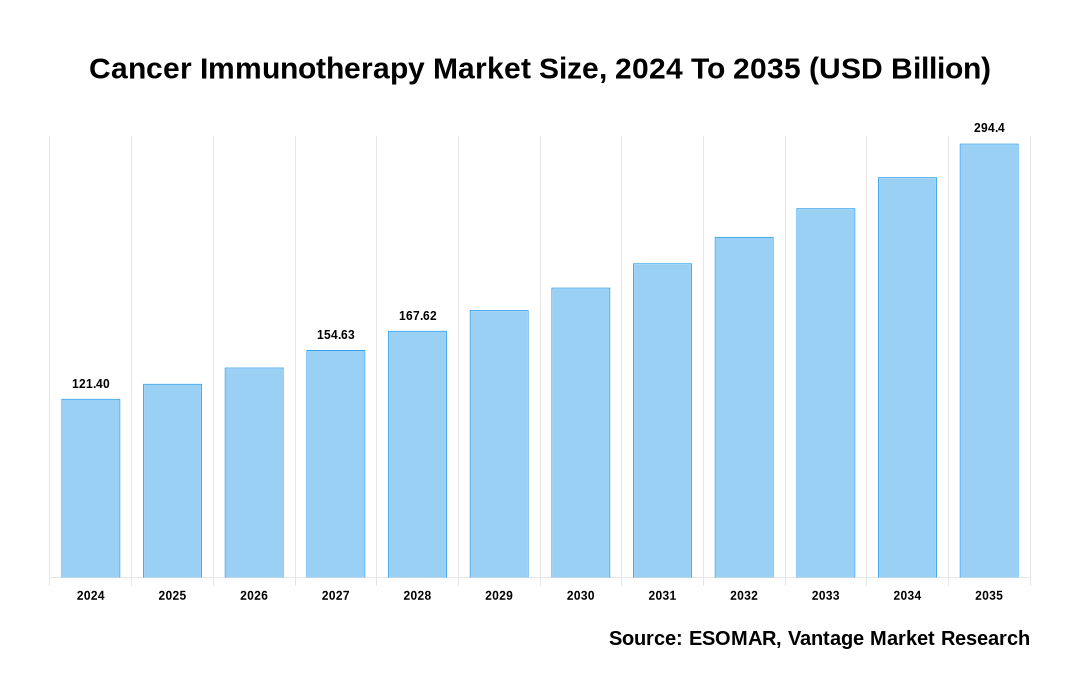
The global Cancer Immunotherapy Market is valued at USD 121.2 Billion in 2023 and is projected to reach a value of USD 245.37 Billion by 2032 at a CAGR (Compound Annual Growth Rate) of 8.15% between 2024 and 2032.
Key highlights of Cancer Immunotherapy Market
- The North American region dominated the market in 2023, obtaining the largest revenue share of 45.1%,
- The Asia Pacific region will witness remarkable growth with a CAGR during the forecast period,
- In 2023, the Monoclonal Antibodies segment dominated the Cancer Immunotherapy market with significant market share of 67.3%,
- The Lung Cancer segment dominated the market with largest market share in 2023,
- In 2023, the Hospital Pharmacy segment dominated the Cancer Immunotherapy market with 59.2% market share,
- The Hospitals & Clinics segment dominated with largest market share of 43.5% in 2023,
- The growing emphasis on personalized medicine and precision oncology approaches creates new opportunities for the development of targeted immunotherapies tailored to individual patients, driving Cancer Immunotherapy market.
Cancer Immunotherapy Market Size, 2023 To 2032 (USD Billion)
AI (GPT) is here !!! Ask questions about Cancer Immunotherapy Market
Cancer Immunotherapy Market: Regional Overview
North America Dominated Sales with a 45.1% share in 2023. North America dominates the market due to various factors including region's advanced healthcare infrastructure, robust research and development activities, and high adoption rates of innovative medical treatments. Particularly in the United States, the forefront of cancer immunotherapy advancements, a well-established ecosystem of leading pharmaceutical companies, academic research institutions, and healthcare providers drives market growth.
Favorable regulatory policies and expedited approval processes by bodies such as the FDA have accelerated immunotherapy drug commercialization in North America. This regulatory framework stimulates R&D spending, fostering creativity and the advent of cutting-edge immunotherapies. The growing incidences of cancer and rising patient focus also support the region's need for cancer treatments inclusive of immunotherapy. for instance, the Canadian cancer Society (CCS) stated approximately 29,800 Canadians recognized with lung cancer in 2020, representing 13% of all new cancer cases, with an estimated 21,200 deaths, comprising 25% of all cancer-associated deaths.
North America's market leadership also stems from its proactive approach to oncology care and early adoption of novel therapies. This proactive approach allows to make sure that immunotherapy is extensively adopted, which solidifies North america's Cancer Immunotherapy market supremacy, together with strong clinical trial infrastructure and patient access applications.
U.S. Cancer Immunotherapy Market Overview
The U.S. Cancer Immunotherapy market, valued at USD 47.26 Billion in 2023 to USD 85.48 Billion in 2032, is anticipated to grow at a CAGR of 6.8% from 2024 to 2032. According to the American Cancer Society journal, approximately 1,958,310 new cases of cancer and 609,820 cancer-related deaths are projected in the United States for the year 2023. The US leads in cancer immunotherapy advances due to a growing number of cancer cases, a strong healthcare system, strong R&D skills, and a supportive regulatory framework. Key players such as Merck & Co. and Bristol-Myers Squibb lead the market, leveraging their expertise and resources to drive innovation and commercialization of novel immunotherapies.
Cancer Immunotherapy Market: Therapy Type Overview
The Monoclonal Antibodies segment dominated the Cancer Immunotherapy market with the largest share of 67.3% in 2023. The Cancer Immunotherapy market, segmented by the Therapy Type, is bifurcated into Monoclonal Antibodies, Immunomodulators and Oncolytic Viral Therapies & Cancer Vaccines.
The monoclonal antibodies reflect the considerable therapeutic effect and widespread adoption of monoclonal antibody-based immunotherapies in cancer remedy. The development of monoclonal antibodies, that are tailor-made to specifically target antigens on cancer cells, has transformed cancer remedy via boosting the immune system's ability to combat tumors while causing the least amount of harm to healthy tissues. Important monoclonal antibody remedies including pembrolizumab, trastuzumab, and rituximab have proven very effective in treating a variety of most cancers’ types, which has caused their significant use and industrial supremacy.
increased investment in the research and development of monoclonal antibodies, such as bispecific antibodies, conjugated monoclonal antibodies, and antigen-binding antibodies, has created new growth opportunities for organizations operating in the field of oncology therapeutics. These next-generation monoclonal antibodies are engineered to confer or exhibit adaptive immunity, antibody-dependent cellular toxicity, and antigen specificity. For instance, in August 2023, the FDA granted approval for Talvey, marking a significant milestone in monoclonal antibody therapy. Talvey is indicated for the treatment of adult patients with relapsed or refractory multiple myeloma who have undergone at least four prior lines of treatment, including proteasome inhibitors, immunomodulatory agents, and an anti-CD38 monoclonal antibody.
The flexibility of monoclonal antibodies' application across multiple cancer indications contributes to their supremacy in the market. Numerous cancers are treated with monoclonal antibody remedy, which include hematological malignancies, colorectal cancer, lung cancer, and breast cancer. Because of their excellent safety profile and effectiveness in treating both solid tumors and hematologic malignancies, monoclonal antibodies are considered cornerstone treatments in modern oncology practice.
Cancer Immunotherapy Market: Government Initiatives
- The initiative "Establishment of Dedicated Services for the Management of Paediatric and Adult Haematolymphoid Cancers in North-East India," introduced under PM-DevINE in Budget 2022-23 with an initial allocation of Rs 1500 crore. This dedicated facility will be located at Dr. B. Borooah Cancer Institute (BBCI) in Guwahati, with an estimated cost of Rs 129 crore. This initiative is poised to significantly enhance cancer care in the region, addressing the needs of the 3,855 childhood and adult Haematolymphoid cancer patients who have sought treatment at BBCI over the past 11 years.
- The Production Linked Incentive (PLI) Scheme, aimed at boosting domestic manufacturing of medical devices, received approval on March 20, 2020. With a total financial outlay of Rs. 3,420 crore, the scheme targets four key segments of medical devices: cancer care/radiotherapy medical devices; radiology & imaging medical devices and nuclear imaging devices; anesthetics & cardio-respiratory medical devices; renal care medical devices and all implants including implantable electronic devices.
- In 2023, the Advanced Research Projects Agency for Health (ARPA-H), a division under the U.S. Department of Health and Human Services (HHS), unveiled a collection of research endeavors valued at more than $330 million. These initiatives are dedicated to fostering biomedical advancements geared towards enhancing healthcare solutions and services for the American populace.
- The government has undertaken various initiatives to promote research in Ayurveda for cancer treatment. The Central Council for Research in Ayurvedic Sciences (CCRAS), established as an autonomous organization, has been actively engaged in cancer-related research, drug development, and documentation of medical practices. Notably, CCRAS has developed AYUSH QOL2C to enhance the quality of life in cancer patients.
- The Partnership for Accelerating Cancer Therapies (PACT) is a collaborative research endeavor spanning five years, jointly initiated by the National Institutes of Health (NIH), the Foundation for the National Institutes of Health (FNIH), and 12 prominent pharmaceutical companies. As a component of the Cancer MoonshotSM Research Initiatives, PACT entails a funding commitment of $220 million.
- President Biden announced the relaunch of the Cancer Moonshot initiative on February 2, 2022, as a key component of the nation's commitment to "end cancer as we know it." Initially spearheaded by President Biden during his tenure as Vice President in 2016, the Cancer Moonshot initiative aims to accelerate progress in the fight against cancer. The renewed initiative sets a national objective of reducing the cancer death rate by at least 50% over the next 25 years, while also enhancing the quality of life for individuals and families affected by cancer.
Key Trends
- A significant development in immunotherapy is the rise of combination regimens that use several different therapeutic modalities, including as CAR-T cell therapy, checkpoint inhibitors, and targeted treatments. These combinations seek to increase the effectiveness of treatment, circumvent mechanisms of resistance, and increase the range of cancer types that can benefit from immunotherapy.
- Precision medicine techniques are becoming more and more popular, as patient classification for individualized care is made possible by the discovery of biomarkers. Selecting immunotherapies based on cancer biomarkers enhances therapeutic results, reduces side effects, and maximizes resource use.
- Predictive modeling techniques and numerous biomarkers are being integrated to improve patient selection and treatment response prediction. More precise treatment classification and treatment response and resistance mechanism prediction are made possible by the integration of omics data, tumor microenvironment studies, and machine learning algorithms.
Premium Insights
According to WHO, in 2022, an estimated, 20 million new instances of cancer and 9.7 million deaths, cancer is the second greatest cause of death. The need for immunotherapy treatments is being further bolstered by the rising incidence of cancer worldwide. The increased prevalence of illness highlights how vital it is to develop new treatment strategies in order to successfully fight cancer, which will propel the worldwide immunotherapy market upward. Pharmaceutical firms' increased efforts in research and development will propel the Cancer Immunotherapy market's projected expansion in the upcoming years. Furthermore, throughout the projected period, technological developments and the launch of novel medications are anticipated to drive market expansion.
The market landscape is evolving with the rising approval of novel immunotherapies, offering promising treatment options for various types of cancer. Recent FDA approvals show tremendous progress in the treatment of cancer, such as quizartinib (Vanflyta) for acute myeloid leukemia (AML). Quizartinib targets FLT3, a kinase that is involved in a large number of AML cases. With its incorporation into induction, consolidation, and maintenance regimens, this therapy represents a significant development in AML treatment protocols, providing a complete strategy to controlling the illness. Furthermore, by encouraging the development of cutting-edge immunotherapies, strategic alliances between market leaders like Immatics and Moderna as well as FBD Biologics Limited and Shanghai Henlius Biotech, Inc. are further stimulating market growth.


Report Coverage & Deliverables
Get Access Now
Track market trends LIVE & outsmart rivals with our Premium Data Intel Tool: Vantage Point
Market Dynamics
Combination therapies involving the simultaneous or sequential use of multiple immunotherapy agents or combining immunotherapy with other treatment modalities offer significant opportunities for enhancing treatment efficacy and expanding treatment options
Checkpoint inhibitors can be used in combination with other immunotherapies, targeted medicines, or conventional treatments like radiation and chemotherapy to boost anti-cancer immune responses, overcome treatment resistance, and increase clinical outcomes. Clinical trials have demonstrated encouraging outcomes for combination medicines, such as higher response rates, longer survival times, and lower toxicity as compared to monotherapy techniques. This has led to an increase in interest in and funding for the creation and assessment of combination immunotherapy regimens for a range of cancer types, offering substantial potential for market expansion and innovation.
Despite the clinical success of immunotherapy, one of the significant barriers to its widespread adoption is the high cost associated with these treatments
The development and manufacturing processes for immunotherapies are complex and resource-intensive, leading to high production costs. The entire cost of immunotherapy is further increased by customized medicine strategies like biomarker testing and patient-specific therapies. Patients are financially burdened by these exorbitant treatment costs, which also restrict therapy access and put a pressure on payers and healthcare systems. In regions with limited healthcare resources or patients facing financial constraints, the high cost of immunotherapy can significantly restrict market penetration and patient access to these life-saving treatments.
There is a vast opportunity for immunotherapies to expand into new indications beyond those currently approved
This includes rare cancers and pediatric cancers, where treatment options are often limited and outcomes are poor. Manufacturers of immunotherapy can meet unmet medical needs and reach niche markets with strong demand for efficient therapies by branching out into new uses. Furthermore, the market size and income potential for producers can be considerably increased by extending the use of immunotherapy to a wider spectrum of cancer types.
Competitive Landscape
The competitive landscape of the Cancer Immunotherapy market is characterized by a dynamic interplay among pharmaceutical giants and innovative biotech firms striving to develop cutting-edge treatments. Established players such as Merck & Co., AstraZeneca, F. Hoffmann-La Roche Ltd, dominate with their pioneering checkpoint inhibitors like Keytruda, Opdivo, and Tecentriq, respectively, which have revolutionized cancer treatment. However, emerging biotech companies like Immunocore and Adaptimmune are gaining traction with their novel T cell receptor therapies, challenging the status quo. This landscape is further enriched by collaborations, mergers, and acquisitions aimed at bolstering pipelines and expanding market reach. The market's rapid evolution, driven by scientific advancements and clinical breakthroughs, underscores intense competition and a continuous quest for innovation to address unmet medical needs in oncology.
The key players in the global Cancer Immunotherapy market include - AstraZeneca (UK), Merck & Co. Inc. (U.S.), F. Hoffmann-La Roche Ltd. (Switzerland), Immunocore Ltd. (UK), Pfizer Inc. (U.S.), Bristol-Myers Squibb Company (U.S.), Novartis AG (Switzerland), Eli Lilly & Company (U.S.), Johnson & Johnson Services Inc. (U.S.) among others.
Recent Market Developments
- In April 2024, GSK plc disclosed that the US Food and Drug Administration (FDA) has approved the supplemental Biologics License Application (sBLA) for Jemperli (dostarlimab). This approval allows for the combination of Jemperli with standard-of-care chemotherapy (carboplatin and paclitaxel) to broaden treatment options for all adult patients dealing with primary advanced or recurrent endometrial cancer, encompassing those with mismatch repair proficient (MMRp)/microsatellite stable (MSS) tumors.
- In October 2023, The U.S. Food and Drug Administration (FDA) has authorized Pfizer's MEKTOVI (binimetinib) + BRAFTOVI (encorafenib) combination for the treatment of adult patients with metastatic non-small cell lung cancer (NSCLC) who have a BRAF V600E mutation as identified by an FDA-approved test.
- In October 2023, The U.S. Food and Drug Administration (FDA) approved Merck's anti-PD-1 drug KEYTRUDA as neoadjuvant treatment and adjuvant treatment after surgery for patients with resectable non-small cell lung cancer (NSCLC).
- In November 2023, The FRUZAQLA (fruquintinib) for Takeda's previously treated metastatic colorectal cancer was authorized by the US FDA.
The global Cancer Immunotherapy market can be categorized as Product, Application, Distribution Channel, End Use, and Region.
| Parameter | Details |
|---|---|
| Segments Covered |
By Product
By Application
By Distribution Channel
By End Use
By Region
|
| Regions & Countries Covered |
|
| Companies Covered |
|
| Report Coverage | Market growth drivers, restraints, opportunities, Porter’s five forces analysis, PEST analysis, value chain analysis, regulatory landscape, technology landscape, patent analysis, market attractiveness analysis by segments and North America, company market share analysis, and COVID-19 impact analysis |
| Pricing and purchase options | Avail of customized purchase options to meet your exact research needs. Explore purchase options |
 Vantage Market
Research | 03-Jul-2024
Vantage Market
Research | 03-Jul-2024
FAQ
Frequently Asked Question
What is the global demand for Cancer Immunotherapy in terms of revenue?
-
The global Cancer Immunotherapy valued at USD 121.2 Billion in 2023 and is expected to reach USD 245.37 Billion in 2032 growing at a CAGR of 8.15%.
Which are the prominent players in the market?
-
The prominent players in the market are AstraZeneca (UK), Merck & Co. Inc. (U.S.), F. Hoffmann-La Roche Ltd. (Switzerland), Immunocore Ltd. (UK), Pfizer Inc. (U.S.), Bristol-Myers Squibb Company (U.S.), Novartis AG (Switzerland), Eli Lilly & Company (U.S.), Johnson & Johnson Services Inc. (U.S.).
At what CAGR is the market projected to grow within the forecast period?
-
The market is project to grow at a CAGR of 8.15% between 2024 and 2032.
What are the driving factors fueling the growth of the market.
-
The driving factors of the Cancer Immunotherapy include
Which region accounted for the largest share in the market?
-
North America was the leading regional segment of the Cancer Immunotherapy in 2023.



