
Biosimilars Market
Biosimilars Market - Global Industry Assessment & Forecast
Segments Covered
By Product Class Monoclonal Antibodies, Recombinant Hormones, Immunomodulators, Anti-Inflammatory Agents, Other Product Classes
By Application Blood Disorders, Growth Hormonal Deficiency, Chronic and Autoimmune Disorders, Oncology, Other Applications
By Region North America, Europe, Asia Pacific, Latin America, Middle East & Africa
Snapshot
| 2022 | |
| 2023 - 2030 | |
| 2017 - 2021 | |
| USD 35.14 Billion | |
| USD 216.32 Billion | |
| 25.50% | |
| Asia Pacific | |
| Europe |
Customization Offered
Cross-segment Market Size and Analysis for Mentioned Segments
Additional Company Profiles (Upto 5 With No Cost)
Additional Countries (Apart From Mentioned Countries)
Country/Region-specific Report
Go To Market Strategy
Region Specific Market Dynamics
Region Level Market Share
Import Export Analysis
Production Analysis
Others Request Customization Speak To Analyst
Market Synopsis:
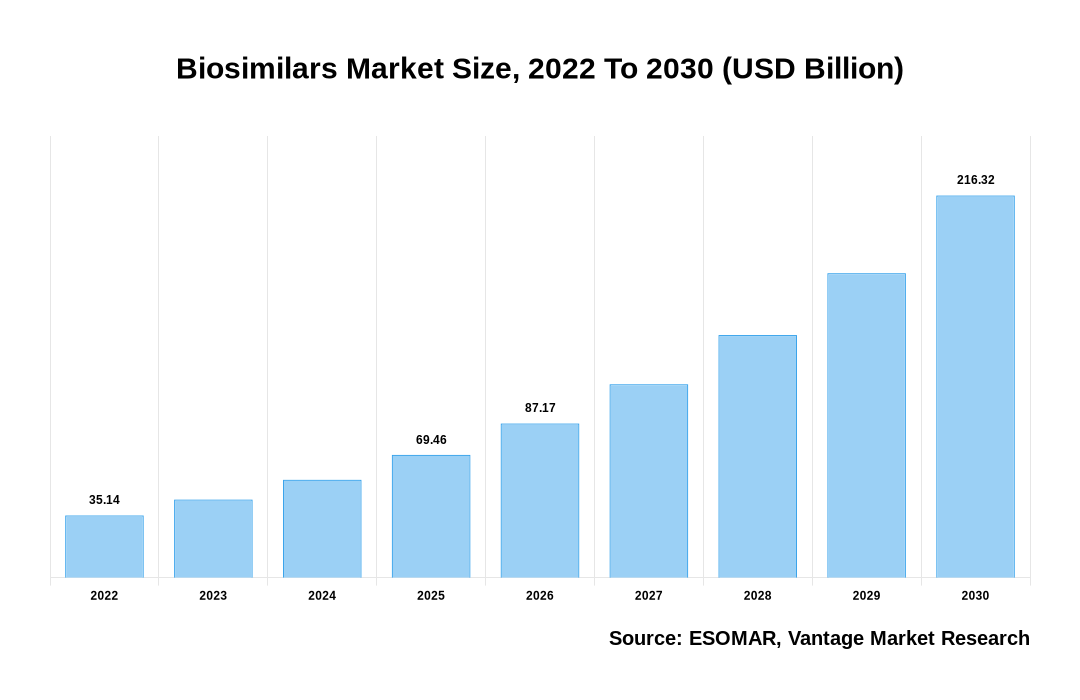
Global Biosimilars market is valued at USD 35.14 Billion in 2022 and is projected to attain a value of USD 216.32 Billion by 2030 at a CAGR of 25.50% during the forecast period, 2022–2028. COVID-19 may have a big impact on the Biosimilars market, and it has provided a considerable barrier to pharmaceutical companies working on Biosimilars development. During the current pandemic, the FDA's approval of non-COVID treatments has been reduced, which is predicted to slow the process of product approval and marketing, stifling market growth. Furthermore, because most clinical studies have been postponed in order to address the COVID-19 crisis and reduce the risk of infection among participants, most pipeline goods are moving at a snail's pace in terms of research and development. There is also a supply chain and raw material shortage as a result of the global lockdown and travel restrictions, which will have an influence on Biosimilars production. COVID-19 has so emerged as a result of the aforementioned circumstances.
The rising frequency of chronic diseases, as well as increased demand for Biosimilars due to their cost-effectiveness, are driving market expansion. Regulatory approvals and other rules in various countries that encourage the use of Biosimilars are also a major driving force in the Biosimilars industry. However, during the forecast period, the complexities of Biosimilars development and manufacturing, as well as resistance from reference biologic manufacturers, are expected to limit the market's growth.
Biosimilars Market Size, 2022 To 2030 (USD Billion)
AI (GPT) is here !!! Ask questions about Biosimilars Market
Increasing Demand for Biosimilar Drugs due to their Cost-Effectiveness
Biosimilars are typically 20–30% less expensive than their branded counterparts. Patients benefit from both cost savings and access to highly effective treatments as a result of this. When compared to originator biologics, biosimilars have cheaper R&D expenses. Biosimilars are less expensive since R&D expenses are reduced. Biosimilars with lower prices have a tendency to drive down the prices of reference biologics due to price rivalry among manufacturers. Biosimilars medications' considerable cost-to-benefit ratio is predicted to increase their demand in the coming years.


Report Coverage & Deliverables
- Real-Time Data Updates:
- Competitor Benchmarking
- Market Trends Heatmap
- Custom Research Queries
- Market Sentiment Analysis
- Demographic and Geographic Insights
Get Access Now
Complexities in Manufacturing
Biosimilars development is a time-consuming and expensive procedure that necessitates major financial inputs, technical capabilities, clinical trial knowledge, scientific standards, and quality control systems. Biosimilars producers, unlike generic drug developers, are obligated to invest in clinical trials and post-approval safety monitoring procedures that are comparable to those of the original innovator businesses. Another significant problem in the production of Biosimilars is the capacity to regulate variability during the manufacturing process, resulting in end products that are identical to their Biosimilars counterparts. In terms of safety and efficacy, Biosimilars should meet established quality requirements.
Market Segmentation:
The Global Biosimilars Market can be segmented by Product Class into Monoclonal Antibodies, Recombinant Hormones, Immunomodulators, Anti-Inflammatory Agents, and Other Product Classes. By Application into Blood Disorders, Growth Hormonal Deficiency, Chronic and Autoimmune Disorders, Oncology, and Other Applications. Based on Region, the Biosimilars Market is segmented into North America, Europe, Asia Pacific, Latin America and Middle East & Africa.
Europe to Continue Dominating the Biosimilars Market
Europe held the greatest proportion of the biosimilars market in 2020, followed by Asia Pacific and North America. The looming patent expiry of biologic drugs and the launch of new biosimilars, the increased frequency of chronic illnesses, the rise of new companies, and early entry into the market are all driving growth in these markets.
Key Players:
Some of the major players in the market are F. Hoffmann-La Roche Ltd. (US), Sandoz International GmbH (Switzerland), Dr. Reddy’s Laboratories Ltd. (India), Teva Pharmaceutical Industries Ltd. (Israel), Pfizer Inc. (US), Samsung Bioepis (South Korea), Biocon (India), and Mylan N.V (US).
The Biosimilars market is segmented as follows:
Parameter
Details
Segments Covered
By Product Class
By Application
By Region
Regions & Countries Covered
Companies Covered
Report Coverage
Market growth drivers, restraints, opportunities, Porter’s five forces analysis, PEST
analysis, value chain analysis, regulatory landscape, technology landscape, patent analysis, market
attractiveness analysis by segments and North America, company market share analysis, and COVID-19
impact analysis
Pricing and purchase options
Avail of customized purchase options to meet your exact research needs. Explore purchase options
The Biosimilars market scope can be tabulated as below:
 Vantage Market
Research | 09-Feb-2022
Vantage Market
Research | 09-Feb-2022
FAQ
Frequently Asked Question
What is the global demand for Biosimilars in terms of revenue?
-
The global Biosimilars valued at USD 35.14 Billion in 2022 and is expected to reach USD 216.32 Billion in 2030 growing at a CAGR of 25.50%.
Which are the prominent players in the market?
-
The prominent players in the market are F. Hoffmann-La Roche Ltd. (US), Sandoz International GmbH (Switzerland), Dr. Reddy’s Laboratories Ltd. (India), Teva Pharmaceutical Industries Ltd. (Israel), Pfizer Inc. (US), Samsung Bioepis (South Korea), Biocon (India), and Mylan N.V (US)..
At what CAGR is the market projected to grow within the forecast period?
-
The market is project to grow at a CAGR of 25.50% between 2023 and 2030.
What are the driving factors fueling the growth of the market.
-
The driving factors of the Biosimilars include
- Increasing Demand for Biosimilar Drugs due to their Cost-Effectiveness
Which region accounted for the largest share in the market?
-
Europe was the leading regional segment of the Biosimilars in 2022.



